The Compliance and Usability Report

What Is Safety-Enhanced Design?
Safety-enhanced design is a method by which implementers demonstrate compliance with certification criteria. This is done through the process of usability testing and feedback – essentially having your users run through the application and getting hands-on with the functionality required by certification criteria.
The results and takeaways from this testing are then reported, often using a template*, and are made publicly available on the ONC CHPL listing for your software. Not only do the results indicate compliance with the certification requirements, but also the practical usability of your software from a user perspective. A minimum of ten participants are required to complete this summative testing, and they will be stepping through all the functionality related to the certification in question. Ensuring compliance is a secondary goal, however, as the primary objective and benefit of this testing is ensuring usability of the implementation!

Challenges of Safety-Enhanced Design
A huge part of the lift involved with creating a safety-enhanced design usability test involves just that, creating the test. The process outlined in your SED will be the same process given to your users, which must be both of the following: expansive enough to cover large concepts and workflows, and granular enough so that users take consistent steps to achieve those expected results. When you then add on factors such as preparing an environment and proctoring the users as they move through the steps, it can add up to a lot of tasks.
We recommend using your workflow process and detailed steps to showcase your software’s usability. This can start with an individual familiar with the software writing a step-by-step process for how they would navigate the workflow in question. Then that can be refined by other team members as they attempt to follow the same steps.
The next challenge involves determining who will follow these steps. Select users who would be likely to use the functionality in their daily workflow. You’ll find that the certification companion guides often outline the requirements for conformance and detail “a limited set of identified users.” Not only do you as the implementer get to define this set of users, but you will also be able to call them here. These could vary from an administrative team, a set of clinicians, a collection of nurse practitioners, or data-entry staff. Remember that the key to this process is determining usability, so selecting the right users who will use the product in the real world is key. An important detail to note is that your workflow may require multiple user groups. Keep this in mind when writing steps so that you account for what each group will be involved with specifically.
The Process
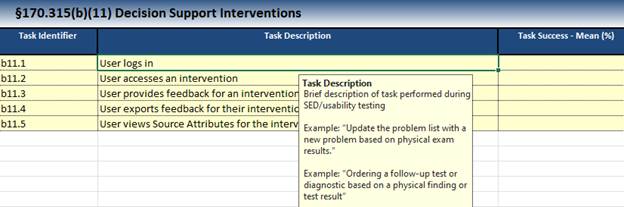
Throughout proctoring the usability steps, you will be recording how users interact with your product, receiving feedback, and drawing conclusions from their actions. Most templates involve recording various data, which can be good to remember when the process is created. Your ACB will provide information for how to complete each field within the template. A few examples are as follows:
- Task success rate
- Task failure rate
- Time it takes for a user to complete a task
- Experience/education/occupation of the user prior to this testing
- User satisfaction or feedback on the functionality
- Significant findings from the test outcomes
- Areas for improvement
The Takeaways
While user testing is a large part of safety-enhanced design, it is only as valuable as the actions taken based on your user feedback. Sure, you will guide the user through the criteria step-by-step, but the metrics of test completion and ease with which the user completes the defined steps will also give you clear takeaways for what else might be needed to complete certification or enhance the software ahead of future requirements. As we have seen recently with (b)(11) supplanting (a)(9) Clinical decision support requirements, certification criteria can change quickly!
Note that your safety-enhanced design is a document that can be amended. In the aforementioned example, if you already have a safety-enhanced design for (a)(9) requirements and are adding (b)(11) functionality, you can follow the same safety-enhanced design format from your ACB and solely focus on new functionality. This will then be listed as an amendment on the CHPL to the existing SED.
Certification Criteria Requiring Safety-Enhanced Design
- § 170.315 (a)(1) Computerized provider order entry (CPOE) – medications
- § 170.315 (a)(2) Computerized provider order entry (CPOE) – laboratory
- § 170.315 (a)(3) Computerized provider order entry (CPOE) – diagnostic imaging
- § 170.315 (a)(4) Drug-drug, drug-allergy interaction checks for CPOE
- § 170.315 (a)(5) Demographics
- § 170.315 (a)(9) Clinical decision support (expires on January 1, 2025)
- § 170.315 (a)(14) Implantable device list
- § 170.315 (b)(2) Clinical information reconciliation and incorporation
- § 170.315 (b)(3) Electronic prescribing
- § 170.315 (b)(11) Decision support interventions
How Can Dynamic Health IT Help?
Consultancy
Dynamic Health IT offers safety-enhanced design consultancy, working to help you create test steps, interpret feedback, and format documentation so that you can share findings with your ACB with confidence. We also offer consultancy specifically for (b)(11) Decision support intervention requirements, especially for applications that are unsure how those requirements overlap with the previous 170.315 (a)(9) Clinical decision support requirements. Our motto is Promoting Interoperability, so if you have any questions or want more information on partnering with us, feel free to reach out at (504) 309-9103.
Public Resources
- Certification Companion Guide for safety-enhanced design – https://www.healthit.gov/test-method/safety-enhanced-design
- NIST common industry format template for usability testing – https://www.nist.gov/publications/nistir-7742-customized-common-industry-format-template-electronic-health-record?pub_id=907312
- The CHPL’s SED directory and information – https://chpl.healthit.gov/#/sed