For EHR vendors, HTI-1 certification deadlines are coming up fast. Here’s your checklist to stay compliant with ONC requirements through 2025 and into 2026:
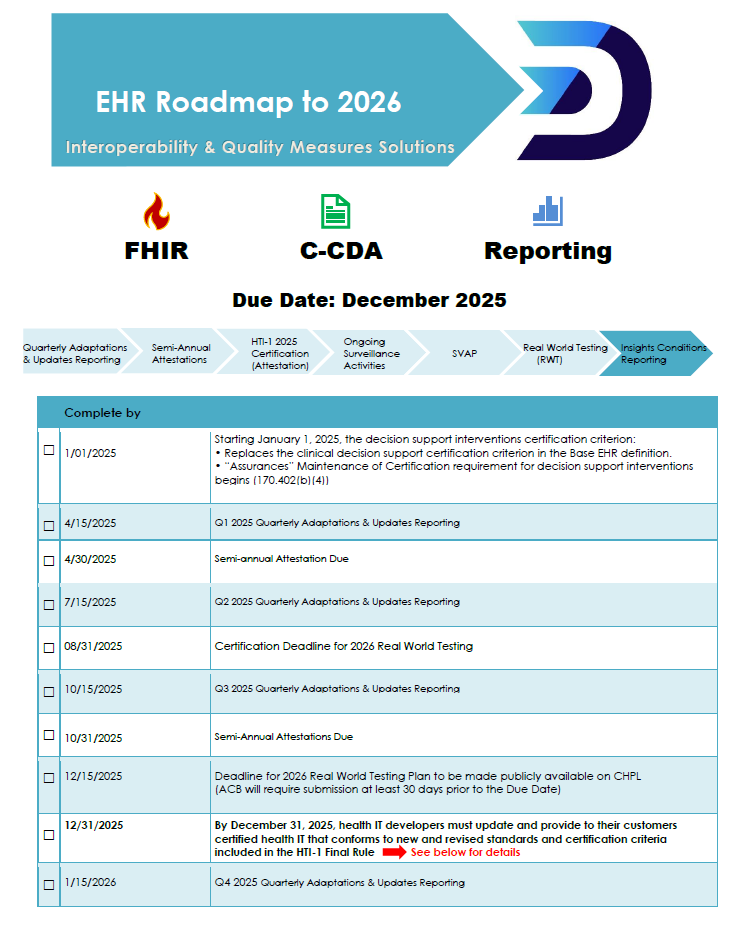
The end-of-year HTI-1 conformance is a heavy lift. Here are some details:
| 1. Your EHR must support new USCDIv3 data elements. 2. CCDs and FHIR API must be updated to these new standards: a. HL7® SMART App Launch Implementation Guide Release 2.0.0 b. HL7 CDA® R2 Implementation Guide: C-CDA Templates for Clinical Notes STU Companion Guide, Release 4.1 c. FHIR US Core IG 6.1.0 d. FHIR R4.0.1 e. Bulk FHIR IG Guide 1.0.1 3. 170.315 (e)(1) change: Patients must be able to restrict sharing of certain classes of data via their patient portal. 4. 170.315 (f)(5) Transmission to public health agencies – electronic case reporting will require bi-directional standards-based electronic care reporting via FHIR or CDA eICR. 5. You’ll need to begin capturing key metrics such as system performance, clinical impact, risk management strategies, and Predictive Decision Support Interventions (PDSI) utilization to satisfy the Insight Condition and Maintenance of Certification Requirements. *** Stay tuned for our next blog for more details! |
And for vendors working on the Patient Demographics requirements for certification, there are changes stemming from Executive Order 14168 signed January 20, 2025 and published in the Federal Register on January 30th: “Defending Women From Gender Ideology Extremism and Restoring Biological Truth to the Federal Government”. These changes apply directly to § 170.315 (a)(5) Patient demographics and observations but affect all criteria that include sending and receiving patient data. So, Certified Health IT modules are no longer required to record:
- Sexual orientation
- Gender identity
- Sex parameters for clinical use
- Name to use, or
- Pronouns
| Contact us for additional information or to schedule a meeting. We are here to help! |