ONC has taken an important step forward with the finalized “Health Data, Technology, and Interoperability” (HTI-1) regulation. Designed to foster interoperability and safe, effective use of AI, HTI-1 supersedes the 2015 Edition Cures Update. Its first provision takes effect March 11, 2024:
- The 170.315(g)(10) Standardized API for patient and population services criterion was revised for patient authorization revocation. The bottom line: All Health IT Modules certified to 170.315(g)(10) must be able to revoke authorized application access within 1 hour of a request and attest to that functionality. Due March 11, 2024.
Whew! Of course, HTI-1 has other provisions, so let’s review the highlights:
- Standards and Functionality Updates
- New Decision Support criteria
- Insights Condition and maintenance of certification requirements
There are also more minor changes to Information blocking regulations that we will address in upcoming blogs. Here are the other changes, listed in chronological order:
FHIR Endpoints
This is another near-term requirement. EHR vendors must publicly publish ‘‘service base URLs’’—also known as ‘‘endpoints’’—for all their customers in a machine-readable format. These URLs make it possible for apps to access EHI at the patient or other authorized party’s request.
Service base URLs must be published in ‘‘Endpoint’’ resource format based on FHIR® Release 4 and US Core IG industry standards. These Endpoint resources must include organization details such as name, location, and provider identifiers (e.g. NPI number). Due: December 31, 2024
New Decision Support criteria
Clinical Decision Support (you’ll remember this one from 2014 and 2015 Edition) gets rebranded as “Decision Support Interventions” in conjunction with its expanded definition. §170.315(a)(9) Clinical decision support (CDS) is replaced by §170.315(b)(11) as ONC expands CDS with an eye on the opportunities and pitfalls presented by artificial intelligence (AI) and machine learning (ML). ONC has imposed transparency requirements for AI and other predictive algorithms that are part of certified health IT.
The transparency requirements aim to clarify how each vendor’s CDI works and specifically, what algorithms are used as the basis of the CDI. The concern is that CDI could be biased in various ways that could lead to recommendations that are ineffective, unsafe or could discriminate against particular population groups.
As of January 1, 2025, b11 will be added to the Base EHR definition and a9 goes away. B11 will be attestation only – no proctor testing. Due: December 31, 2024
Standards and Functionality HTI-1 Updates
Note that these are all due by December 31, 2025:
- United States Core Data for Interoperability Version 3 (USCDI v3) – compared with v1 there are:
- 47 new elements plus 30 elements with updates to vocabulary standards
- Five new classes
- Five elements moved to different classes
- Two deleted and three renamed classes
- Health IT Modules certified to criteria that reference USCDI must update the following criteria to USCDI v3 using the applicable US Core IG and C-CDA Companion Guide:
- § 170.315(b)(1): Transitions of Care
- § 170.315(b)(2): Clinical Information Reconciliation and Incorporation
- § 170.315(b)(9): Care Plan
- § 170.315(e)(1): View, Download, and Transmit 3rd Party
- § 170.315(g)(6): Consolidated CDA Creation Performance
- § 170.315(g)(9): Application Access-All Data Request
- Various code systems have been updated, including SNOMED, RxNorm, LOINC and NDC.
- In addition to USCDIv3, vendors certified for the 170.315(g)(10) FHIR API are responsible for the following:
- Update APIs to HL7® FHIR® US Core Implementation Guide STU 6.1.0
- Implement HL7® SMART App Launch Implementation Guide Release 2.0.0, including mandatory support for the “Capability Sets” of “Patient Access for Standalone Apps” and “Clinician Access for EHR Launch”; all “Capabilities” as defined in “8.1.2 Capabilities,” excepting the “permission-online” capability; “Token Introspection” as defined in “7 Token Introspection”
- HL7® CDA® R2 Implementation Guide: C-CDA Templates for Clinical Notes STU Companion Guide, Release 4.1
- Electronic Case Reporting – ONC is moving toward newer CDA and FHIR standards. EHR vendors can choose either HL7 CDA or HL7 FHIR implementation guides and eCR Now is a popular choice for vendors leveraging the FHIR standard.
Insights Condition and Maintenance of Certification
This is a new twist from ONC. They are requiring EHR vendors to take responsibility for calculating and reporting utilization for these measures:
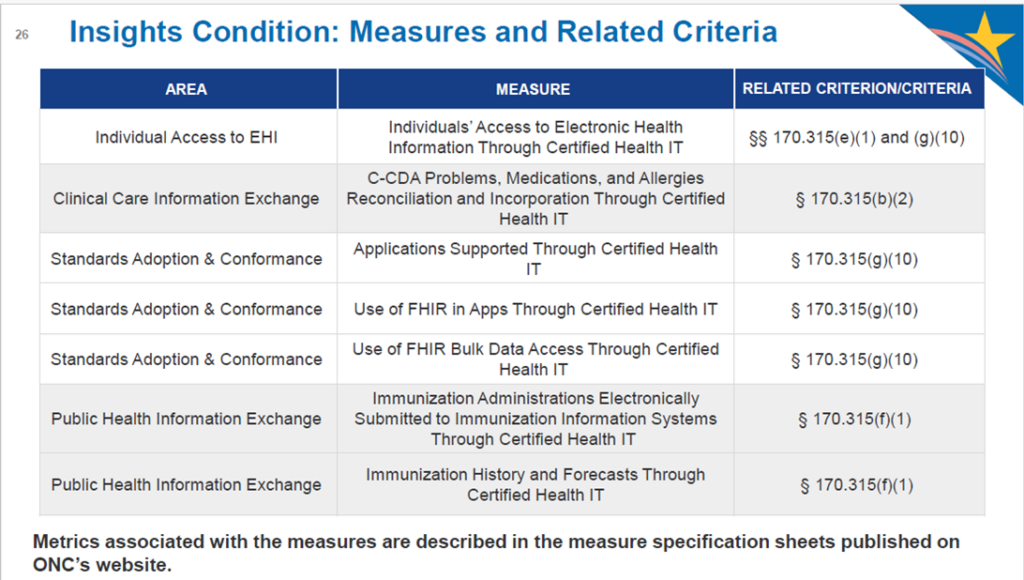
Smaller vendors with less than 50 client hospital sites and less than 500 clinician users are exempt and will just need to attest to the exemption. Also exempt would be EHR vendors without any users of the particular criterion. Data will have to be aggregated across all of a vendor’s client sites and submitted via web services. ONC will be providing templates that vendors can use for submitting this data.
Vendors will need to collect data over the course of a calendar year (starting January 1, 2026) and then compile and submit to ONC. ONC has a multi-year timeline, with a data gathering reporting year, followed by 6 months to assemble the data and report. The good news for vendors is that the metrics you compile from Insights Condition can be leveraged to meet RWT requirements. Contact DHIT for more information about our RWT services.
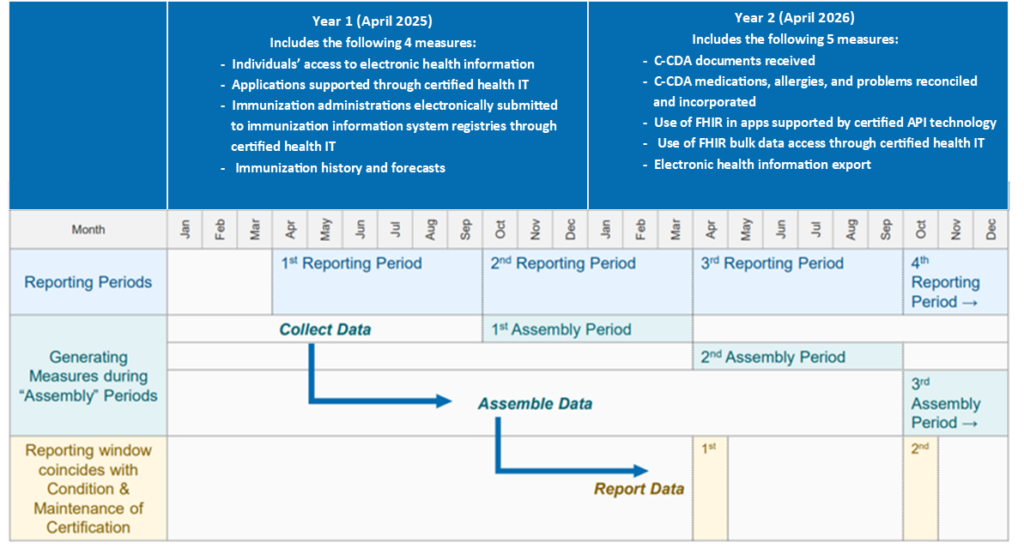
The following measures will be implemented in Year 1:
- Individuals Access to EHI Through Certified Health IT
- Applications Supported Through Certified Health IT
- Use of FHIR in Apps Through Certified Health IT
- Immunization Administrations Electronically Submitted to Immunization Information Systems Through Certified Health IT
ONC will make the results publicly available – interesting! DHIT will continue to monitor and blog about the HTI-1 rule as deadlines loom and will dive deeper into specific requirements that affect our customers. Stay tuned!

