What you Need to Know
Like most of our EHR partners, DHIT is focused on the 170.315 (b)(10) end of year compliance deadline (see past blogs). While that’s a big, important deadline, it’s also important to keep an eye on what the longer-term future holds. With that in mind, we encourage you to review the new “Health Data, Technology, and Interoperability” (HTI-1) Proposed Rule. Since it is still a proposed rule, the ONC is soliciting your feedback through June 20th. This is your opportunity to potentially influence the final rule.
Here’s a quick recap of ONC’s HTI-1 Proposed Rule, along with our (non-biased, of course) feedback. To start with, you’ll notice that ONC is moving away from their previous naming convention. If you recall, we had the 2011 Edition, the 2014 Edition, and then the 2015 Edition which stuck around for a long, long time. After feedback from Health IT developers, ONC determined, “we believe there should be a single set of certification criteria, which will be updated in an incremental fashion in closer alignment to standards development cycles and regular health IT development timelines.”
For EHR vendors, the highlights are:
- Standards and Functionality Updates
- New Decision Support criteria
- Conditions and maintenance of certification requirements
Standards and Functionality Updates
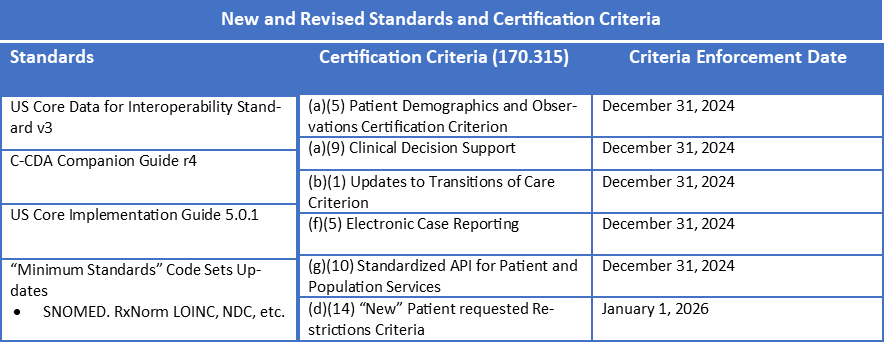
- USCDI requirements advance from v1 to v3 with accompanying US Core and C-CDA updated companion guides. These updates include SDOH and Payer Data, along with required code systems for other data.
- Health IT Modules certified to criteria that reference USCDI must update to USCDI v3 by the end of 2024 using the applicable US Core IG and C-CDA Companion Guide:
- § 170.315(b)(1): Transitions of Care
- § 170.315(b)(2): Clinical Information Reconciliation and Incorporation§ 170.315(b)(9): Care Plan
- § 170.315(e)(1): View, Download, and Transmit 3rd Party
- § 170.315(g)(6): Consolidated CDA Creation Performance
- § 170.315(g)(9): Application Access-All Data Request
- Various code systems have been updated in the proposed rule, including SNOMED, RxNorm, LOINC and NDC.
- In addition to USCDIv3, 170.315(g)(10) FHIR API will be updated for USCDIv3, US Core and SMART v2. Also, an updated version of the FHIR standard will be required – either FHIR US Core Implementation Guide STU version 5.0.1 or, if finalized, US Core IG v6.0.0.
Patient Requested Restrictions
ONC is introducing a new “Patient Requested Restrictions” Criterion — §170.315(d)(14). Basically, it is a required new capability for an EHR user to be able to flag restricted data on a patient-by-patient basis and then prevent this data from being shared. In addition, patients (and their authorized representatives) must be able to use an internet-based method to restrict sharing of any USCDIv3 data. Presumably, this could happen via a patient portal and/or FHIR API and individual 3rd party App Consents.
Per the proposed rule, it would be required by January 1, 2026 (or 24 months after the effective date of a final rule). Reading the proposed rule, it is not clear what level of granularity will be required. It appears that ONC is aiming for more granularity than the typical “Opt in/Opt out” HIE reporting model, but would it be OK to flag/restrict broad categories of data for a particular patient (e.g. restrict procedures or restrict problems) or do we have to go to the level of the individual problem or procedure (e.g. don’t share this patient’s abortion procedure).
The proposed rule repeatedly talks about this new criterion being “standards agnostic”, but it also mentions the current “security tags—send” 170.315(b)(7) and (b)(8) and security tags—receive as being functionality that could be leveraged at the developer’s discretion. It appears that Provenance could also be a tie-in. It will be interesting to see how this one plays out!
New Decision Support criteria
Clinical Decision Support (you’ll remember this one from 2014 and 2015 Edition) gets rebranded as “Decision Support Interventions” in conjunction with its expanded definition. §170.315(a)(9) Clinical decision support (CDS) will eventually be replaced by §170.315(b)(11). In the interim period between publication of the final rule and the deadline to comply, §170.315(a)(9) will be added to the Real World Testing requirement. With §170.315(b)(11), ONC intends to expand CDS with an eye on the opportunities and pitfalls presented by artificial intelligence (AI) and machine learning (ML). In addition, ONC is proposing that b11 be added to the Base EHR definition.
Insights Condition and Maintenance of Certification
This is a new twist from ONC. Currently, vendors collect various utilization statistics as directed by the 170.315(g)(2) Automated Measures criterion and then Providers who participate in MIPS and hospitals who participate in Promoting Interoperability submit the metrics to CMS. With this new Insights requirement, ONC proposes to leverage some of these existing metrics, add some new metrics, and require EHR vendors to report utilization directly to ONC. Here’s the list of the measures:

Additionally:
- Data will have to be submitted via a web-based form and made publicly available every six months with reporting phased in over two years
- Smaller vendors with less than 50 client hospitals and less than 500 clinician users would be exempt, although they would still have to report that exempt status.
- Also exempt would be EHR vendors without any users of the particular criterion.
- Vendors will have to roll up data from multiple versions/releases of their products.
- Interesting!
See proposed timeline, below:

To make your voice heard, you may submit comments, identified by RIN 0955-AA03, through June 20th, 2023 at http://www.regulations.gov and you can upload attachments, too. Read more of the Proposed Rule. ONC has hosted a series of webinars explaining the proposed rule, https://www.healthit.gov/news/events/hti-1-proposed-rule-information-sessions.
At DHIT, we welcome your feedback on requirements and are eager to assist with certification. Our expert consulting and bolt-on modules have paved the way for many successful EHR vendor certifications. Please contact Michelle Bond for additional information, mbond@dynamichealthit.com.

