Hospitals
DHIT has over 20 years of experience supporting hospitals
Dynamic Health IT has solutions for Promoting Interoperability, patient engagement and quality measure reporting, including submission through Inpatient Quality Reporting (IQR) and The Joint Commission.
DHIT partners enjoyed a 100% success rate in Hospital submissions for the IQR program
Quality Measure Reporting
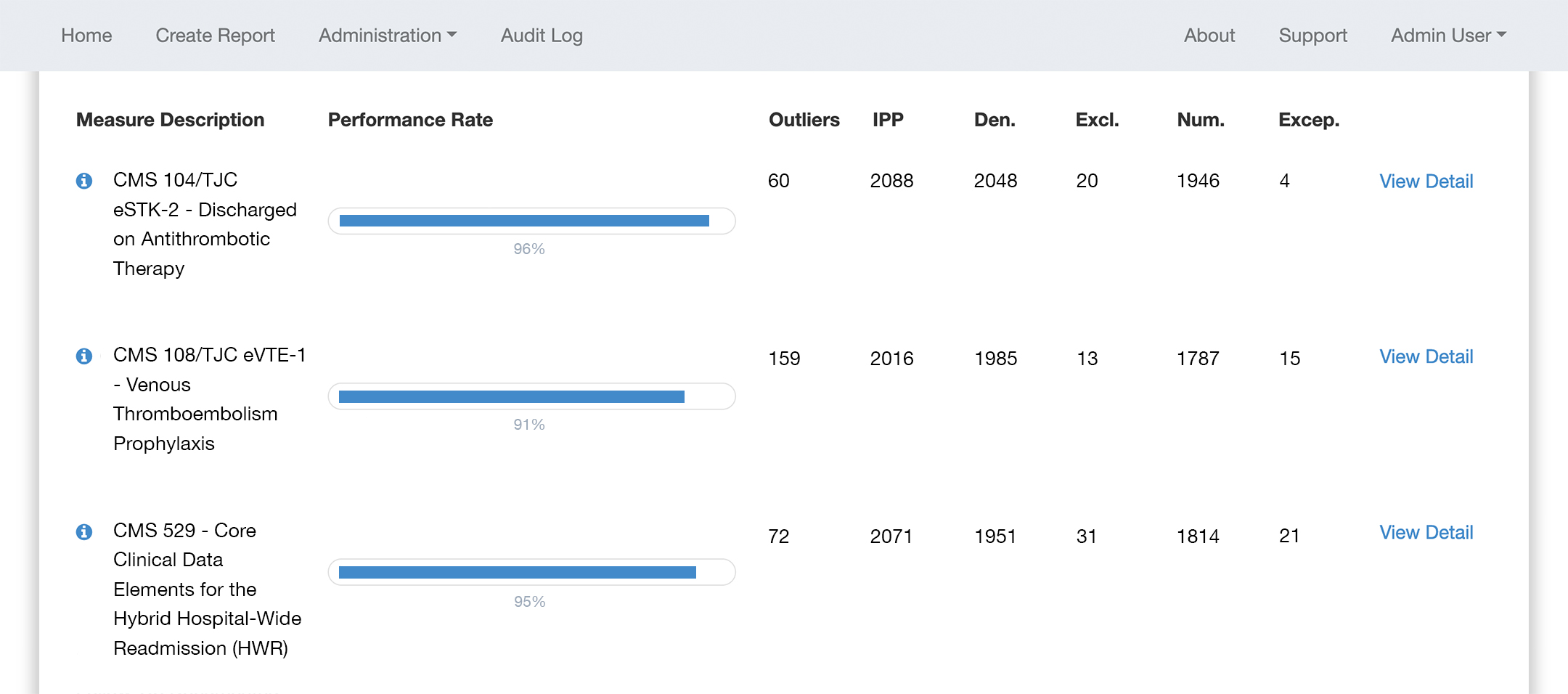
DHIT has Quality Measure software to support:
Inpatient Quality Reporting
For the CY 2024 reporting period/FY 2026 payment determination, hospitals must:
- Report a total of six eCQMs. Hospitals are required to submit the Safe Use of Opioids, Cesarean Birth, Obstetric Complications eCQMs and self-select three additional eCQMs from the measure set.
- Report four quarters of data for six eCQMs using EHR technology certified to the Office of the National Coordinator (ONC) for Health Information Technology’s existing 2015 Edition Cures Update criteria.
Fulfilling the Hospital IQR Program eCQM requirement also satisfies the clinical quality measure reporting requirement for the Medicare Promoting Interoperability Program. CY 2024 reporting will apply to FY 2026 payment determinations for subsection (d) hospitals.
For more information, please refer to the Electronic Clinical Quality Measure (eCQM) Overview web page on QualityNet and the eCQI Resource Center website (https://ecqi.healthit.gov).
Hospitals must submit eCQM data via the Hospital Quality Reporting Secure Portal by February 28, 2025.
CQMsolution supports all IQR eCQM and Hybrid measures.
For the CY 2024 reporting period/FY 2026 payment determination and subsequent years hospitals may use a third-party vendor, such as DHIT to submit QRDA Category I files on their behalf or self-report through HQR Secure Portal in QualityNet.
With the latest ONC certification, CQMsolution is the ideal tool to satisfy eCQM reporting requirements. CQMsolution outputs QRDA I files specifically formatted for submission to QualityNet.
The Joint Commission updated the 2024 ORYX® Performance Measure reporting requirements effective January 1, 2024. These updates affect reporting requirements for Joint Commission–accredited critical access hospitals and hospitals. Key updates include:
- Health Care Organizations (HCOs) that are required or elect to submit eCQMs are required to submit eCQM data for a minimum of all four quarters, applicable to the services provided and patient populations served.
- If an HCO is required but is unable to submit eCQM data, the HCO must submit an extenuating circumstance request (ECR) form prior to the chart-abstracted deadline.
- HCOs must submit a new ECR form for each year and in no case may an HCO be granted an exception for more than five consecutive years.
In 2024, accredited hospitals must submit both electronic clinical quality measure (eCQM) and chart-abstracted measure data via The Joint Commission’s Direct Data Submission Platform (DDSP). There are staggered start and end dates, as shown on The Joint Commission web site.
CQMsolution supports all eCQMs for the latest edition of ONC Certification, and that includes all eCQMs submitted to The Joint Commission (JHACO).
DHIT’s extensive quality measure reporting experience enables us to assist our clients with reporting eCQMs as part of the overall hospital accreditation process. As a Data Submission vendor, not only can we calculate and compile eCQMs from your EHR data but we can electronically submit for you.
Promoting Interoperability

DHIT promotes Interoperability to support:
Patient Engagement

DHIT has Patient Engagement software to support:
The Interface Engine
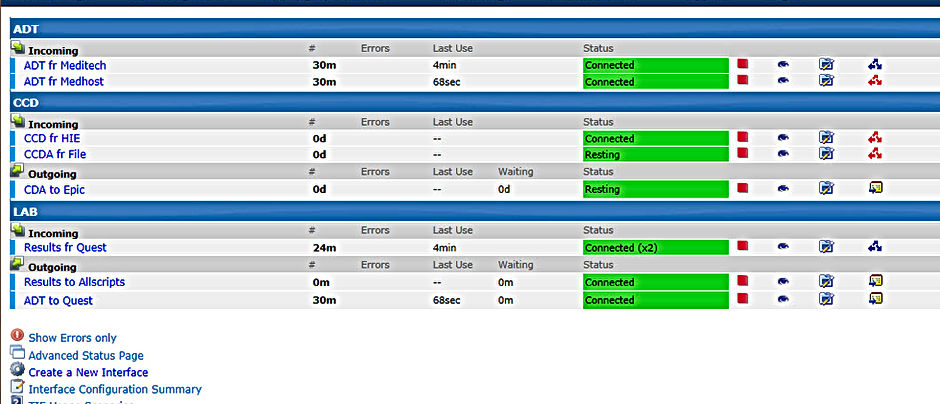
DHIT’s TIE® (The Interface Engine) software is an HL7® & Clinical Document Architecture engine offering the best combination of affordability, ease of use and processing power on the market
Example Use Cases:
- Bi-Directional HIE Connections (ADT, Lab & Imaging Results, C-CDA)
- ADT Event Notifications
- RxNT
- Bi-Directional Immunization
- All HL7v2 transformation and Transport


