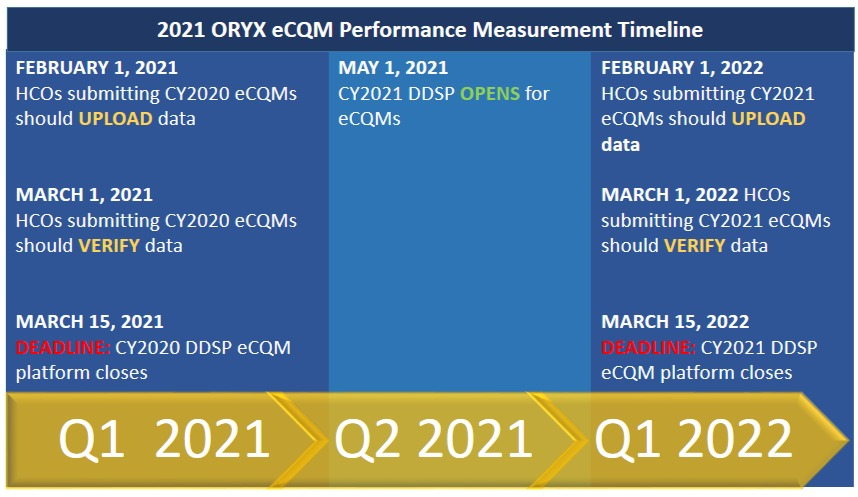
Now that 2020 is in the rearview mirror, let’s take a look at the TJC ORYX 2021 timeline for Healthcare Organizations (HCOs):

We’ve already blogged about eCQM IQR requirements so here’s a quick overview of Joint Commission ORYX requirements. As a data submission vendor and a previous ORYX vendor, we are known as the quality measure experts. Here are the key takeaways for 2021:
- Critical Access Hospitals must report data
- You must submit the same measures for 2 quarters of data (changed from 1 quarter)
- Additional eCQMs are available
- eCQMs (equivalents) may be submitted instead of the Perinatal Care chart-abstract measures
- Deadline is March 15, 2022, using the DDS platform
New eCQM Measures for 2021
Similar to IQR requirements, you must submit two-quarters of data and quarters do not have to be consecutive. You must submit the same measures in each quarter, so make sure you are monitoring measure scores and choosing wisely. No measures have been removed or retired for CY2021 but there are 2 new measures:
- Unexpected Complications in Term Newborns (ePC-06) (Joint Commission Only Measure)
- Safe Use of Opioids – Concurrent Prescribing (eOPI-1) (IQR & Joint Commission)
NOTE: For any of the Perinatal Care measures ( PC-01, PC-02, PC-05, and PC-06) hospitals may submit a minimum of two-quarters of eCQM data (ePC-01, ePC-02, ePC-05 and ePC-06) instead of four quarters of the corresponding chart-abstracted measures.

For Chart-Abstracted measures or more information on reporting requirements, 2021 ORYX Reporting Requirements
Data Submission Vendor
Healthcare organizations that exceed the minimum ORYX eCQM requirements and satisfy eligibility criteria will receive recognition through the Pioneers in Quality program. It is essential to select the appropriate Strategic Partner for 2021 to effectively monitor performance scores, provide guidance in measure selection, and ensure successful submission. Following The Joint Commission’s elimination of “self-reporting” for ORYX data, healthcare organizations must now submit either abstracted data or electronic clinical quality measures (eCQMs) through the Direct Data Submission (DDS) platform. We recommend partnering with a qualified third-party expert to reduce administrative burden and streamline the submission process.